How To Given an explicit formula, write the first n terms of a sequence Substitute each value of n into the formula Begin with n=1 to find the first term, a1 To find the second term, a2 , use n=2 Continue in the same manner until you have identified all n terms See also what is day of the dead called in spanish Electrocyclic Reactionswoodward Hoffmann Rule For 4n And (4n 2) Pi Systems this video explains the experimental results of electrocyclic reactions involving 4n and (4n 2) pi systems with the help of woodward hoffmann rule this video dr norris reviews the woodward hoffmann rules for electrocyclic reactions and does some example problems the following videos containHave one p orbial per atom of the ring;
A Disrotatory 4n 2 Electron Anti Aromatic Mobius Transition State For A Thermal Electrocyclic Reaction Henry Rzepa S Blog
4n+2 rule example
4n+2 rule example-The much greater stabilization in "aromatic" conjugated rings, and Hückel's 4n2 rule, derive from alternating stabilization and destabilization of successive orbitals when the ends of a conjugated chain overlap as it is closed to form a ring A circle mnemonic predicts orbital energies for conjugated ringsFor some reason none of my organic chemistry textbooks, lectures, etc explain what N actually is, so I made this video




Aromaticity A Brief Discussion All Bout Chemistry
This organic chemistry video tutorial shows you how to tell if a compound is aromatic, antiaromatic or nonaromatic by using huckel's rule / number of 4n2 pi So you take all the pi electrons and lone pairs (when applicable to be included) and set them equal to 4n2 electrons For example the number 10 is a huckel number because 4n2=10 n=2 and as long as n=0,1,2 (an integer) it passes the test Upvote 0The (4n2) rule is a consequence of the degeneracy of the π orbitals in cyclic conjugated hydrocarbon molecules As predicted by Hückel molecular orbital theory, the lowest π orbital in such molecules is nondegenerate and the higher orbitals form degenerate pairs For benzene the lowest π orbital is nondegenerate and can hold 2 electrons, and the next 2 π orbitals form a
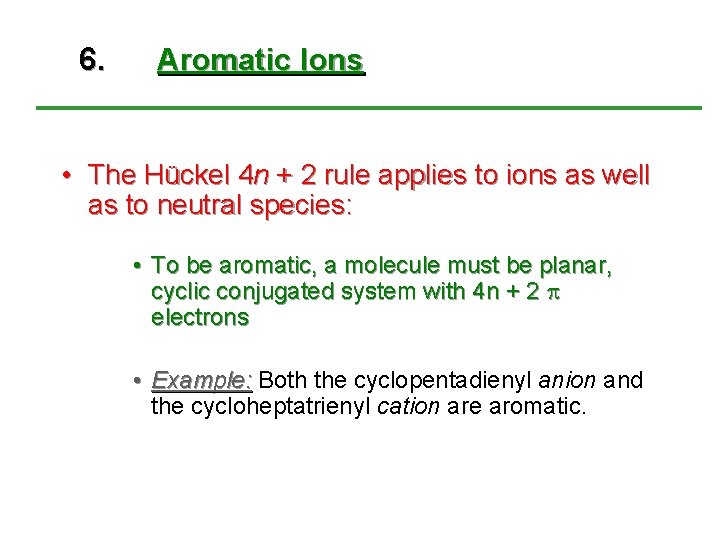
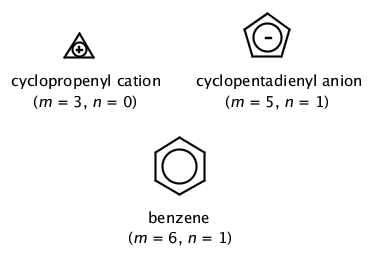
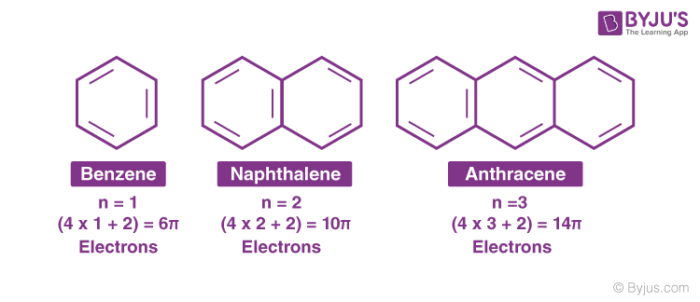
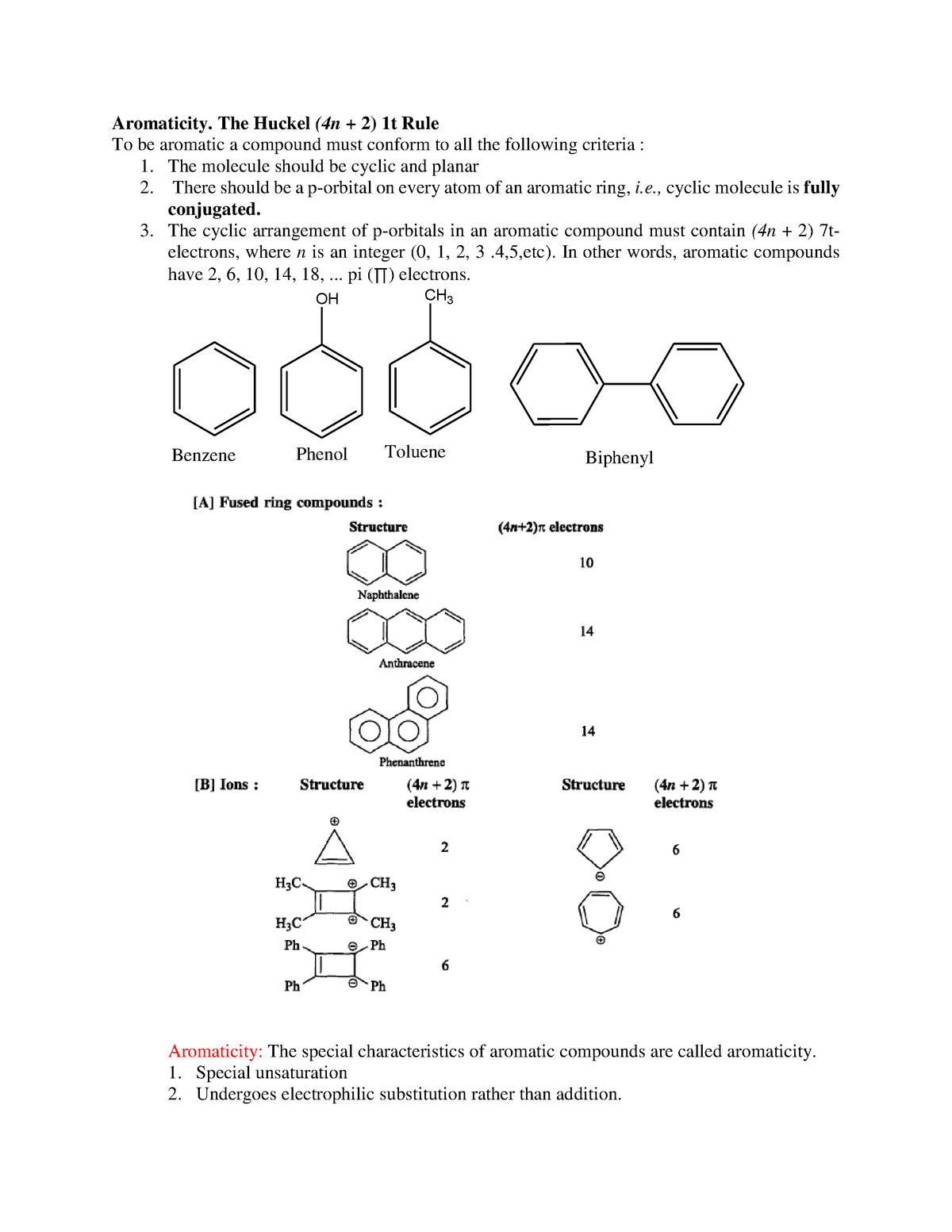
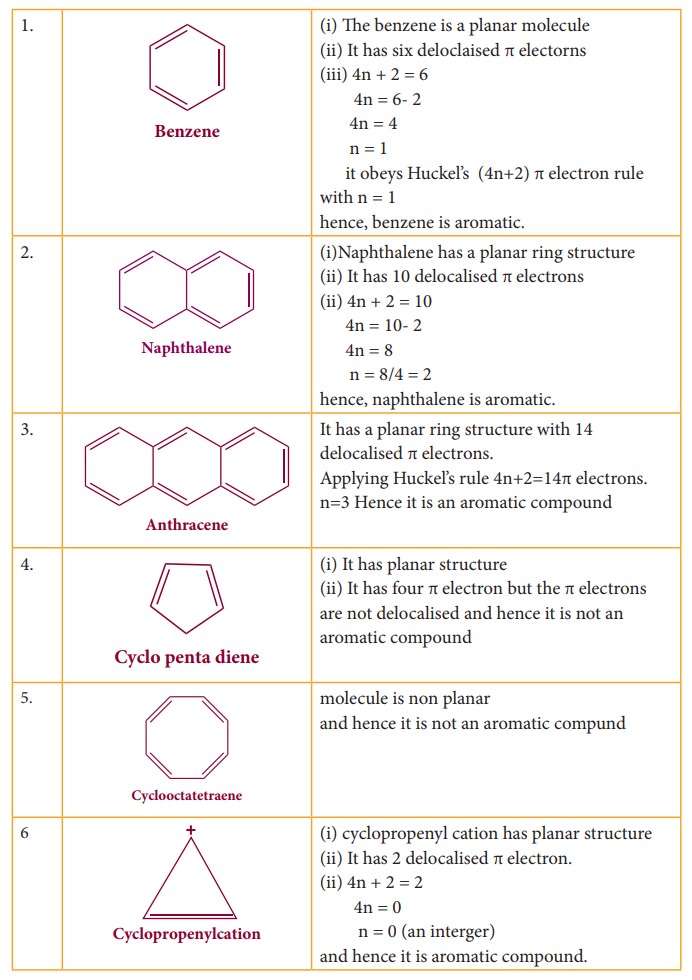
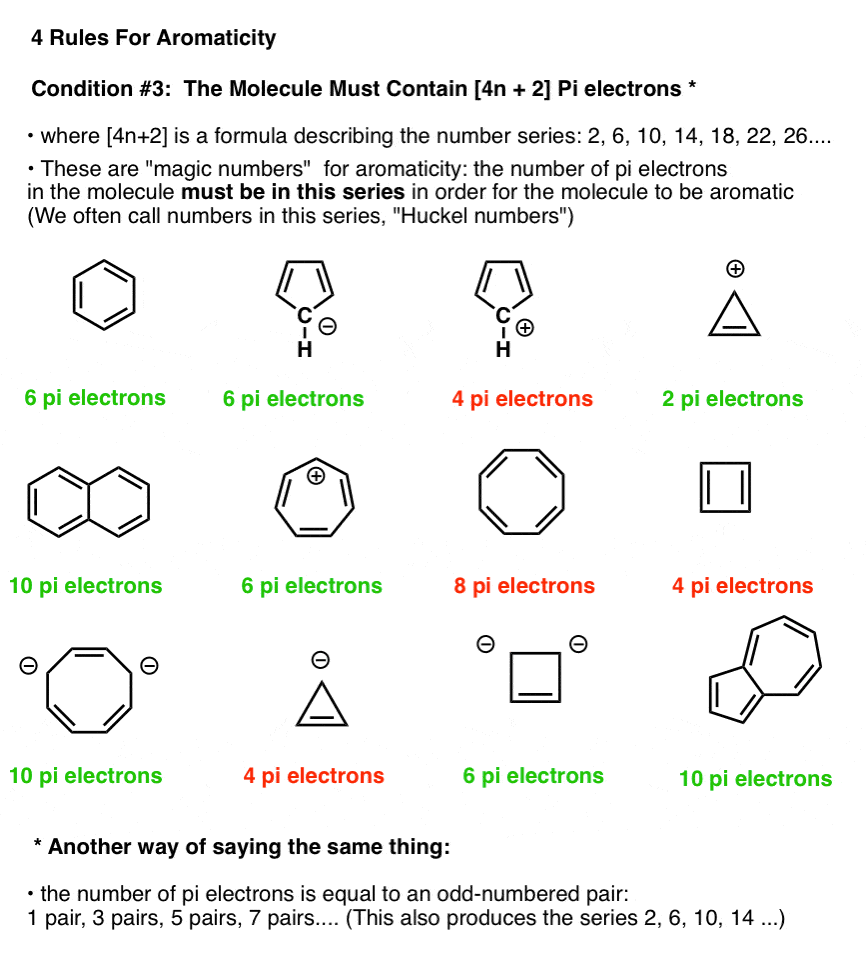
If we put n=2 in the Huckel's rule, we get, 4n2 = 4×22 = 10 = pielectrons in the molecule Naphthalene molecule has a ring system, ie, it is a cyclic molecule The ring system contains pielectrons that are fully delocalized and all the atoms present are sp 2 hybridized It is a planar molecule Ques 3The ring systems having the following characteristics are aromatic (i) Planar ring containing conjugated π bonds (ii) Complete delocalization of the π electrons in ring system ie, each atom in the ring has unhybridised porbital , and (iii) Presence of (4 n 2) π electrons in the ring where n is an integer (n = 0, 1, 2) Huckel rule Using this information classifies the followingHückel's Rule also applies to ions As long as a compound has 4n2 π electrons, it does not matter if the molecule is neutral or has a charge For example, cyclopentadienyl anion is an aromatic ion
4 and 8 can be generated by the formula 4n where n is an integer LMSweeting Jan 03 Cyclic, planar, 14π e – s obeys Hückel`s (4n2) π (n=3) rule for aromaticity Now pK a values increases from cyclopentadiene to indene ie, acidity gradually decreases This phenomenon can be explained by so called `annulation` process whereby some rings in fused systems give up part of their aromaticity to adjacent ringFactor 4n^24n1 4n2 − 4n 1 4 n 2 4 n 1 Rewrite 4n2 4 n 2 as (2n)2 ( 2 n) 2 (2n)2 − 4n1 ( 2 n) 2 4 n 1 Rewrite 1 1 as 12 1 2 (2n)2 − 4n12 ( 2 n) 2 4 n 1 2 Check that the middle term is two times the product of the numbers being squared in the first term and third term 4n = 2⋅(2n)⋅1 4 n = 2 ⋅ ( 2 n) ⋅ 1




Aromatic Compounds Huckel S Rule 4n 2 Pi Electrons Youtube



15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts
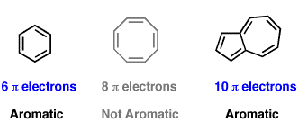
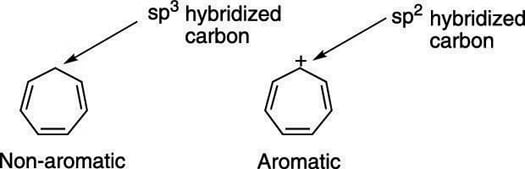
For example, pyrene contains 16 conjugated electrons (8 bonds), and coronene contains 24 conjugated electrons (12 bonds) Both of these polycyclic molecules are aromatic even though they fail the 4n 2 rule Indeed, Hückel's rule can only be theoretically justified for monocyclic systems Pyrene is a commonly used counterexample of Hückel's This gives us 6 total pi electrons, which is a Huckel number (ie satisfies 4n2) What is Huckel rule with example?Be planar, in an sp2 hybridized orbital, over every atom of the ring;



Organic Chemistry Chem Ppt Download



Open Questions In Boron Species With Globally 4n P Systems Communications Chemistry
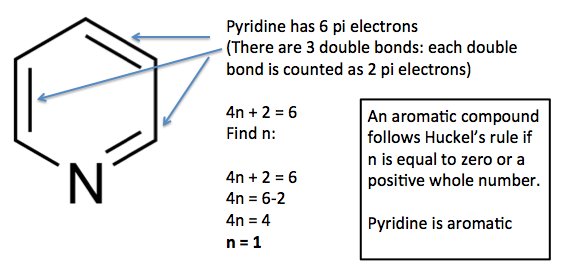
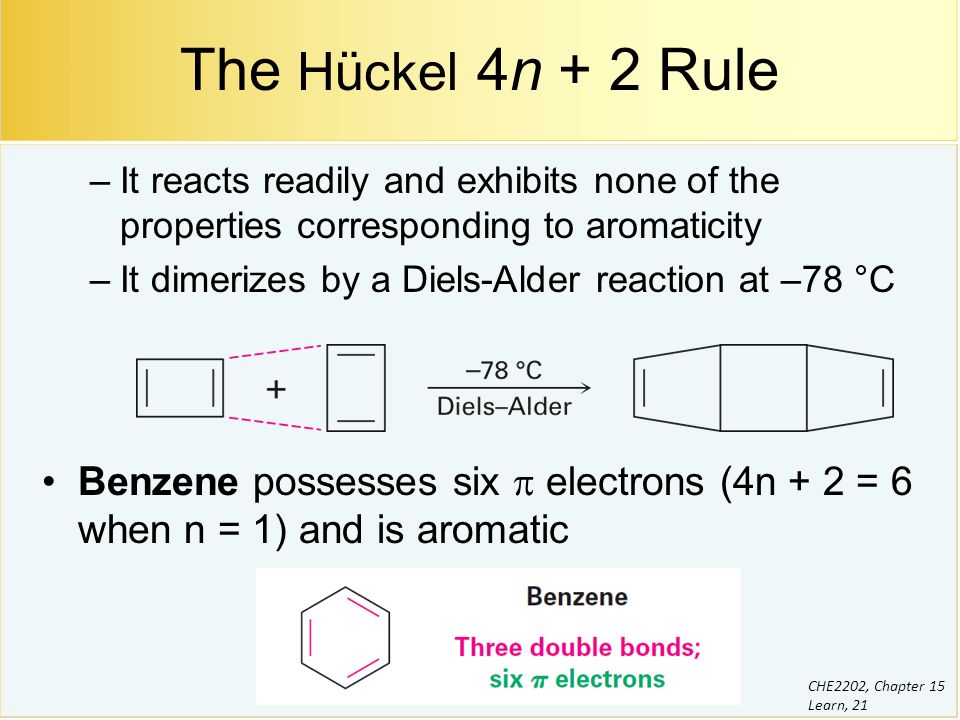
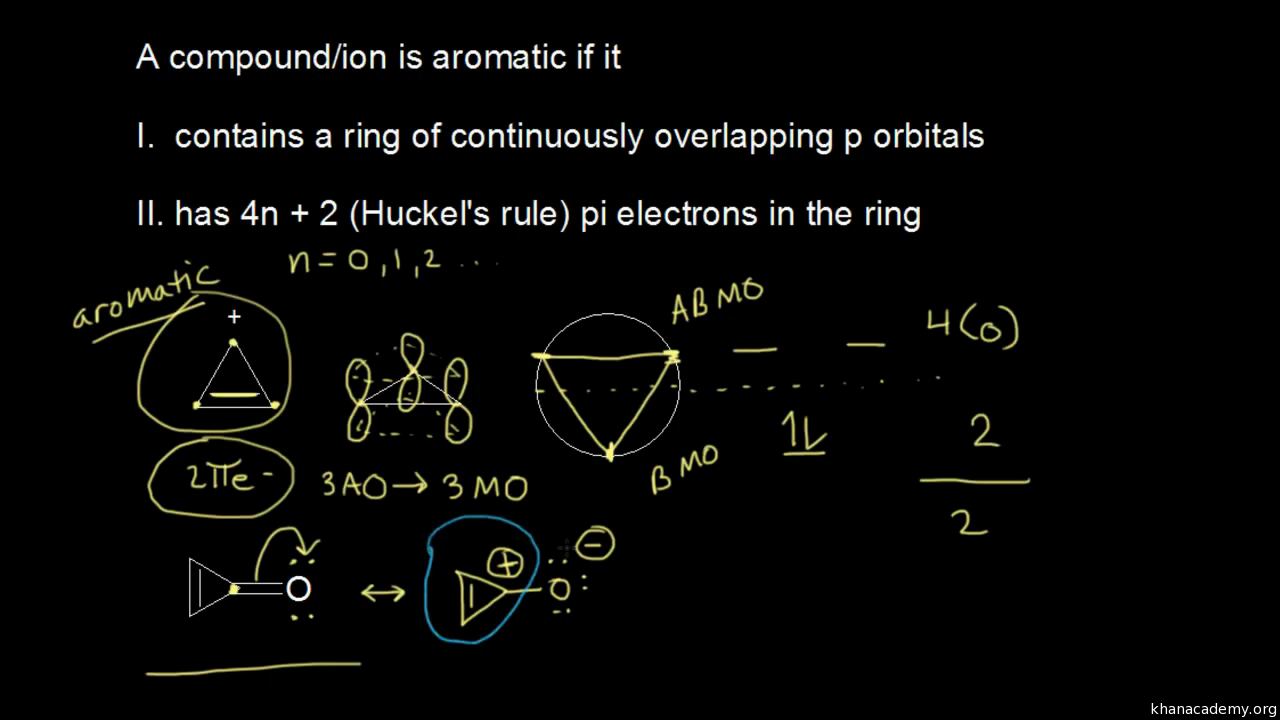
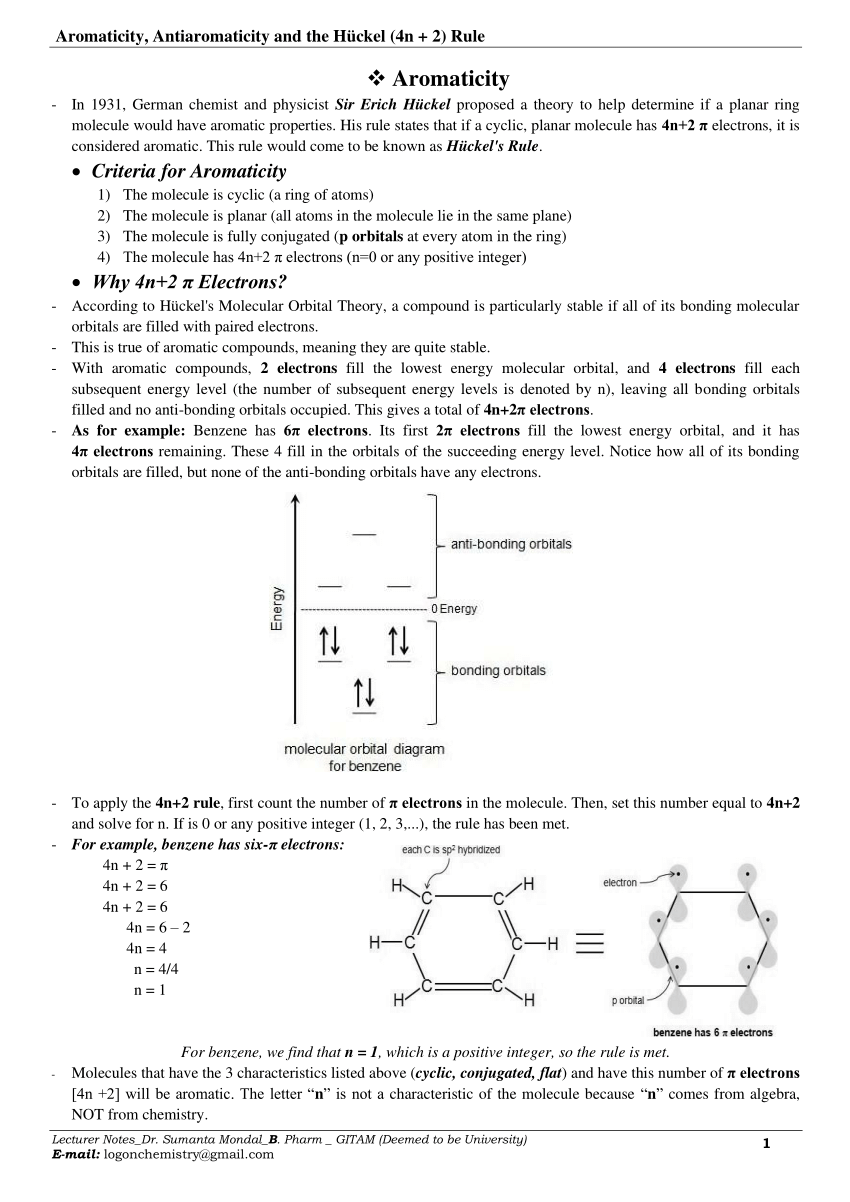
To apply the 4n2 rule, first count the number of π electrons in the molecule Then, set this number equal to 4n2 and solve for n If is 0 or any positive integer (1, 2, 3,), the rule has been met For example, benzene has six π electrons 4n 2 = 6 4n = 4 n = 1 The Huckel aromaticity rules are Molecule is cyclic; In polycyclic conjugated hydrocarbons the Hückel (4n 2)‐rule may be violated, so that certain (4n 2)‐membcred rings cause thermodynamic destabilization The structural conditions for the occurrence of such an effect are analyze d Eight examples (of which seven are new) of non‐b enzenoid hydrocarbons are put forward, in which the Hückel rule is disobeyed



Solved Question 12 Dalered Dared Cee Using The Menu Select The Best Term To Match The Definition Description Example Benzene Said To Be Aromatic Because Is Planar Cyclic All Atoms Within The Ring




A Closer Examination Of Huckel S Rule And The Polygon Rule
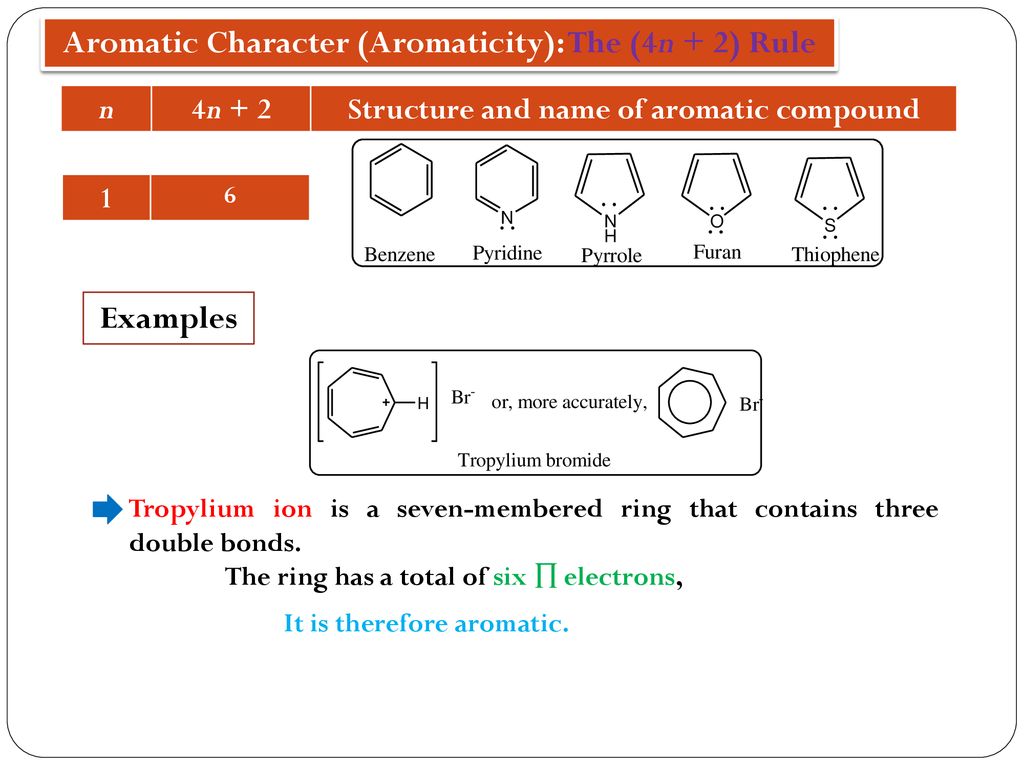
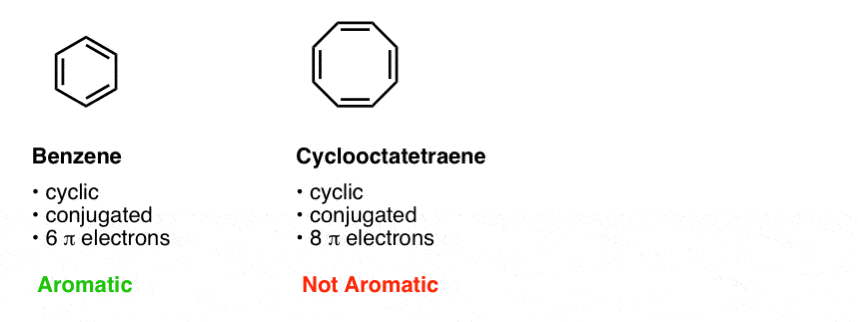
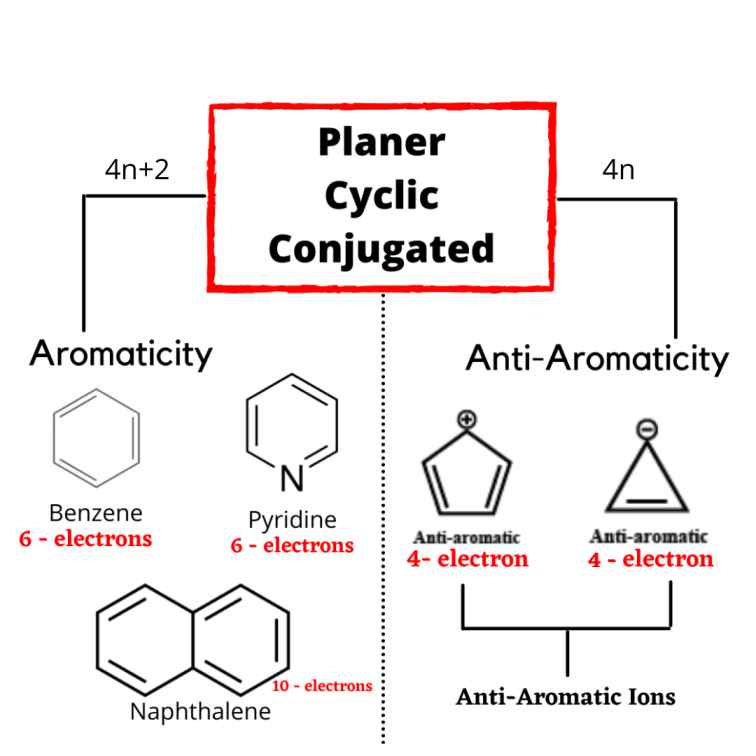
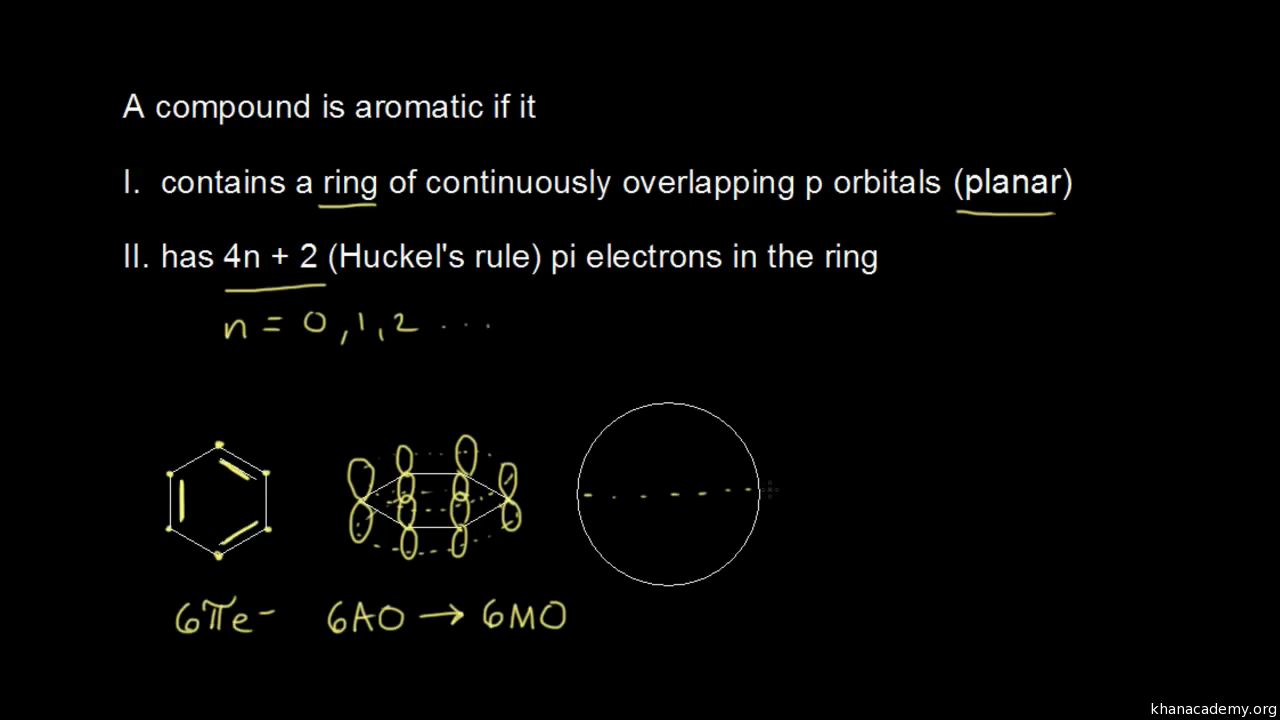
N is just any natural number which is used to satisfy the 4n 2 rule If that number becomes equal 4n 2 for any value of n then that compound is aromatic(or in other words if the number of pi electrons come in the series – 2, 6, 10, 14, 18 then that compound will be aromatic) Note that "n" in Huckel's Rule just refers to any whole number, and 4n2 should result in the number of pi electrons an aromatic compound should have For example, 4 (0)2 gives a twopielectron aromatic compoundHuckel's 4n2 Rule Huckel's 4n2 rule is used to determine if a planar, cyclic, conjugated system has aromatic character or stabilization A molecule is typically considered aromatic if it planar, cyclic, conjugated and has 4n2 π electrons where n=0, 1, 2, 3 etc For example benzene which is planar, cyclic, conjugated and has 4n2=6 π electrons (ie n=1) and therefore is aromatic



Aromatic Stability Iii Video Khan Academy




What Is An Aromatic Compound Definition Example Video Lesson Transcript Study Com
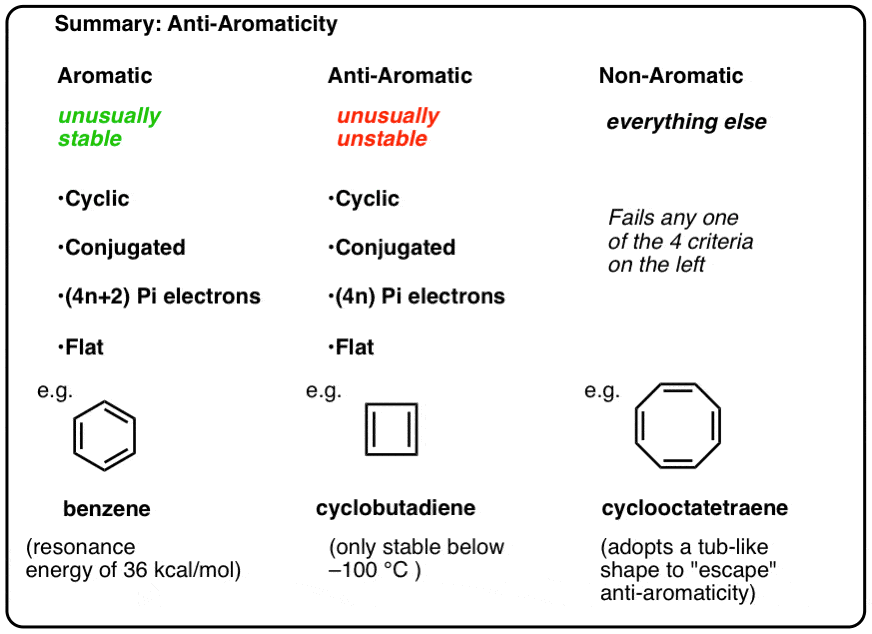
Generated from the formula 4n2 where n is an integer Those which have 4 and 8 electrons have two electrons unpaired and in nonbonding orbitals (same energy as the porbitals) and thus have much less bonding (two electrons are not bonding) and are very reactive;Illustrated Glossary of Organic Chemistry Huckel's Rule (4n2 rule) In order to be aromatic, a moleculemust have a certain number of pi electrons(electrons with pi bonds, or lone pairswithin porbitals) within a closed loop of parallel, adjacent porbitals The pi electroncount is defined by the series of numbers generated from 4n2 where n = zero or any positive integer (ie, n = 0, 1, 2,The rule is generally limited to n = 05 This rule is derived from the Hückel MO calculation on planar monocyclic conjugated hydrocarbons (CH)m where m is an integer equal to or greater than 3 according to which (4n 2) electrons are contained in a closedshell system Examples of systems that obey the Hückel rule include
.jpg?revision=1)



15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts




Aromatic Stability I Video Khan Academy
n is just any natural number which is used to satisfy the 4n 2 rule 1 Count the number of pi electrons 2 If that number becomes equal 4n 2 for any value of n then that compound is aromatic(or in other words if the number of pi electrons come in the series – 2, 6, 10, 14, 18 then that compound will be aromatic)Aromatic compounds contain 4n2 π electrons, where n is a whole number starting from 0 This is called the Hückel's rule discovered by Erich Hückel in 1931 For example, Benzene has 6 π electrons and it satisfies the Hückel's rule since the n, in this case, is equal to one Number of electrons = 4 x 1 2 = 6A general rule can be established for suprafacialsuprafacial cycloadditions For i = 1, 2, 3 and 4i = m n, or 4i 2 = m n where m and n are even numbers, then the following Table applies This Table can be generalized even more but this would take us beyond the scope of this exercise Fig 10




What Is An Aromatic Compound Definition Example Video Lesson Transcript Study Com



13 6 Aromaticity Chemistry Libretexts
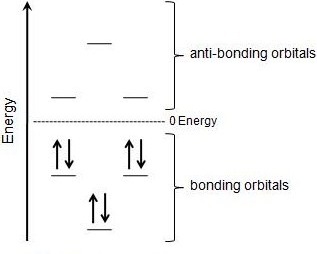
If is 0 or any positive integer (1, 2, 3,), the rule has been metFor example, benzene has sixπ electrons 4n 2 = π 4n 2 = 6 4n 2 = 6 4n = 62 4n = 4 n = 4/4 n = 1 For benzene, we The 5 bonding orbitals contain 2 pairs of degenerate orbitals and along with tex\psi/tex 1 tex\psi/tex 1 can contain 2 electrons, while each degenerate pair has a capacity of 4 electrons Thus, the rule 4n2, which is the configuration having all pibonding orbitals completely filled, associated with extra stability Let's take an example For solving same problem, you have two functions f(n) =4n^2 2n4 and g(n) =4n4 For f(n) =4n^22n4 so here f(1)=424 f(2)=1644 f(3)=3664 f(4)=6484 As you can see here contribution of n^2 is a quadric function whose value will increase with increase in value of n




Huckel Rule Or 4n 2 Rule For Aromaticity Examples And Exceptions Youtube




Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry
Example 2 sequence with a term to term rule of 1 We subtract 1 from the first term to give the next term in the sequence, and then repeat this to generate the sequence We can work out previous terms by doing the opposite of the term to term rule Step by step guide Arithmetic Sequence 2 Geometric sequencesPentalene is an example of an antiaromatic compound that has been well studied experimentally for years It is dicyclic, planar, and has eight πelectrons Anionic and dicationic cases of Pentalene are aromatic since they follow Huckel's 4n 2 πelectron rule State the Difference between Aromatic, NonAromatic, and AntiAromatic CompoundsHückel's 4n 2 rule ′hu̇k·əlz ¦fȯr ‚en pləs ′tü ‚rül (organic chemistry) Aromatic (ring) compounds must have 4 n 2 pibonding electrons, where n is a whole number and generally limited to n = 0 to 5 When n = 1, for example, there are six pielectrons, as for benzene Also known as Hückel's rule




Aromatic Antiaromatic Or Nonaromatic Huckel S Rule 4n 2 Heterocycles Youtube



Structure Aromaticity And Huckels Rule
It follows the same 4n2 rule as for benzene, but this time counting the electrons involved in the reaction rather than just the πelectrons in the aromatic ring The advantage of this approach is that aromaticity is quite stable to perturbations to the symmetry (benzene rings in eg cyclophanes for example can be bent by up to 30° and stillExample, in Rh6(CO)16 the total number of electrons would be 6 × 9 16 × 2 − 6 × 10 = 86 – 6 × 10 = 26 Therefore, the cluster is a closo polyhedron because n = 6, with 4n 2 = 26 When structure prediction is To apply the 4n2 rule, first count the number of π electrons in the molecule Then, set this number equal to 4n2 and solve for n If is 0 or any positive integer (1, 2, 3,), the rule has been met For example, benzene has six π electrons



Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry




What Is Huckel S Rule 4n 2
The succinct expression as the 4n 2 rule has been attributed to Wv E Doering (1951), although several authors were using this form at around the same timeAccording to this rule, if there are (4n2)Pi electrons (and not bonds) delocalized in all the atoms of the corresponding molecule then it's an aromatic molecule Example 15K views ·With the 4n 2 rule 'n' needs to be any whole number Let's look at what our options for the number of electrons are based on this rule n = 1 4(1) 2 = 6 ;



Rules For Aromaticity The 4 Key Factors Master Organic Chemistry



What Is 4n 2 P Rule Quora
N = 3 4(3These numbers are consistent with Hückel's 4n 2 rule of aromaticity 27, 70,71 On the other hand, Table 3 The average numerical results of ring carbon atoms for eight informationtheoretic What is N in 4n 2 rule?



Chapter 15 Introduction In Early 19 Th Century




Huckel S Rule 4n 2 Rule 4n 2 Huckel S Rule 4n 2 Rule Aromaticity Youtube
How does the 4n2 Rule Work?N = 2 4(2) 2 = 10 ;Factor n^24n4 n2 − 4n 4 n 2 4 n 4 Rewrite 4 4 as 22 2 2 n2 − 4n22 n 2 4 n 2 2 Check that the middle term is two times the product of the numbers being squared in the first term and third term 4n = 2⋅n ⋅2 4 n = 2 ⋅ n ⋅ 2 Rewrite the polynomial n2 − 2⋅n⋅222 n 2



Iupac Huckel 4 Em N Em 2 Rule H



Illustrated Glossary Of Organic Chemistry Term
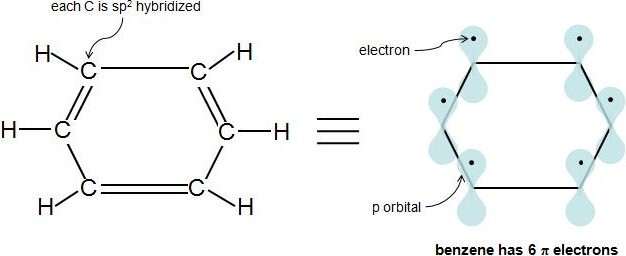
What is N in the 4n2 rule?Q The 4n2 Rule is also sometimes called the Huckel Rule Recall that n can be 0,1,2,3, so that the systems which are highly stabilized have 2,6,10,14,pi electrons in a cyclic conjugated system q By the way, the 4n2 Rule is not applicable at all to acyclic conjugated systems, where 4n2 electron systems are no more or less stable than 4nThe ring obeys the Hückel's rule, which states that the number of electrons in the loop of latex \pi /latex electrons is equal to 4n 2, where n is zero or a positive whole number As is evident from the above examples, determining whether a ring obeys the Hückel's rule or not using the Lewis diagram is straightforward



What Are Aromatic Compounds Socratic



Properties Of Aromatic Compounds Chemistry Revision Site
So Huckel's Rule for Both Aromatic and AntiAromatic is same but only differ in formula (4n2)& (4n)Other criteria 1,2,3 are same for both 1It must be Planer Molecule/ion 2It must be cyclic molecule/ion 3It shouldn't have any sp3 carbon in the ring In short words, we can simply say that the aromatic compound and antiaromaticHuckel's Rule (4n2 rule) In order to be aromatic,a molecule must have a certain number of pi electrons within a closed loop of parallel, adjacent p orbitals The pi electron count is defined by the series 4n2 where n = zero or a positive integer (0, 1, 2, etc) The most common examples are Furan, Pyrolle, Naphthalene, Anthracene etcFew Examples of Huckel's Rule Cyclopropenyl Anion 4π Electrons having a planer conjugated system (4n,n=1) 2 Electrons one double bond 2 Electrons lone pair of carbon with a negative charge Therefore, it is AntiAromatic Cyclopropenyl Cation 2π with a planer conjugated system(neither 4n nor 4n2) 2 Electronsone double bond




Huckel S Rule Explanation Of Huckel S 4n 2 Rule To Estimate Aromaticity



Sigmatropic Rearrangements Huckel Mobius Approach
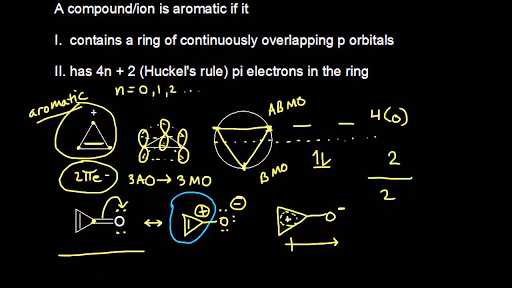
The bestknown example is benzene (C6H6) with a conjugated system of six π electrons, which equals 4n 2 for n '=' 1 Why cyclopentadienyl anion is aromatic?Answer (1 of 2) Hückel's Rule states that if the number of electrons in the cyclic system is equal to (4N2), where N is a whole number integer, then the system is aromatic If the number of electrons in the cyclic system is equal to 4N, where N is a whole number integer, then the system isHave a closed loop of 4n2 pibond electrons, where n is equal to any integer (0,1,2,3,) But like most natural phenomenon, there exists a rule exactly the opposite



Aromatic Compounds Definition Example Properties Nomenclature With Videos



How To Determine The Aromaticity Of A Ring System Dummies
The cyclic cyclopentadienyl anion is planar, it possesses a cyclic uninterrupted π electron cloud, and it meets Hückel's rule, as it has 4*1 2 (n '=' 1) π electrons




Aromatic Compounds Benzene Its Family Nanoplasmonic Research Group Organic Chemistry Chapter 4 Part I Ppt Download



1



Aromaticity Lecture Notes 11 Aromaticity The Huckel 4n 2 1t Rule To Be Aromatic A Compound Studocu



Benzene And Aromaticity Ppt Video Online Download



Aromatic Hydrocarbons Nomenclature And Isomerism Aromaticity Structure Sources Chemistry




Warm Up Write The First Five Terms Of An 4n 2 A1 4 1 Ppt Download



1



How To Identify Aromatic Anti Aromatic Non Aromatic Compounds




Connecting And Combining Rules Of Aromaticity Towards A Unified Theory Of Aromaticity Sola 19 Wires Computational Molecular Science Wiley Online Library



Aromaticity Rules And Definition Organic Chemistry Help




A Closer Examination Of Huckel S Rule And The Polygon Rule



Aromatic Stability Iii Video Khan Academy



What Is The Example Of An Aromatic Compound Without Hydrogen Quora




Aromaticity A Brief Discussion All Bout Chemistry



Antiaromaticity And Antiaromatic Compounds Master Organic Chemistry
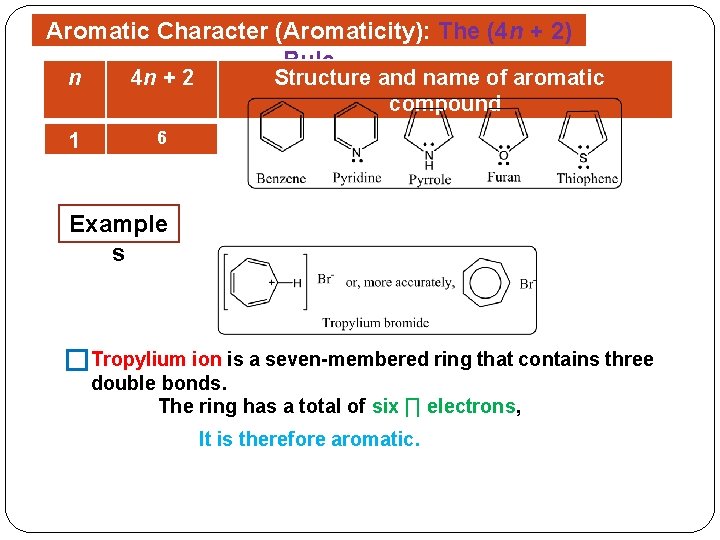



Organic Chemistry Chem 145 Chemistry 2 Credit Hrs
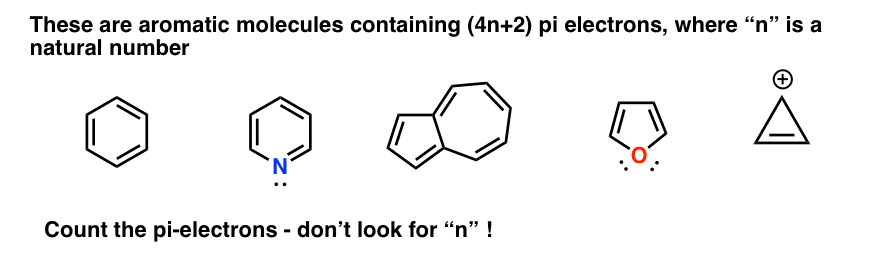



Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry



Anti Aromaticity Avoided A Tutorial Example Henry Rzepa S Blog



2
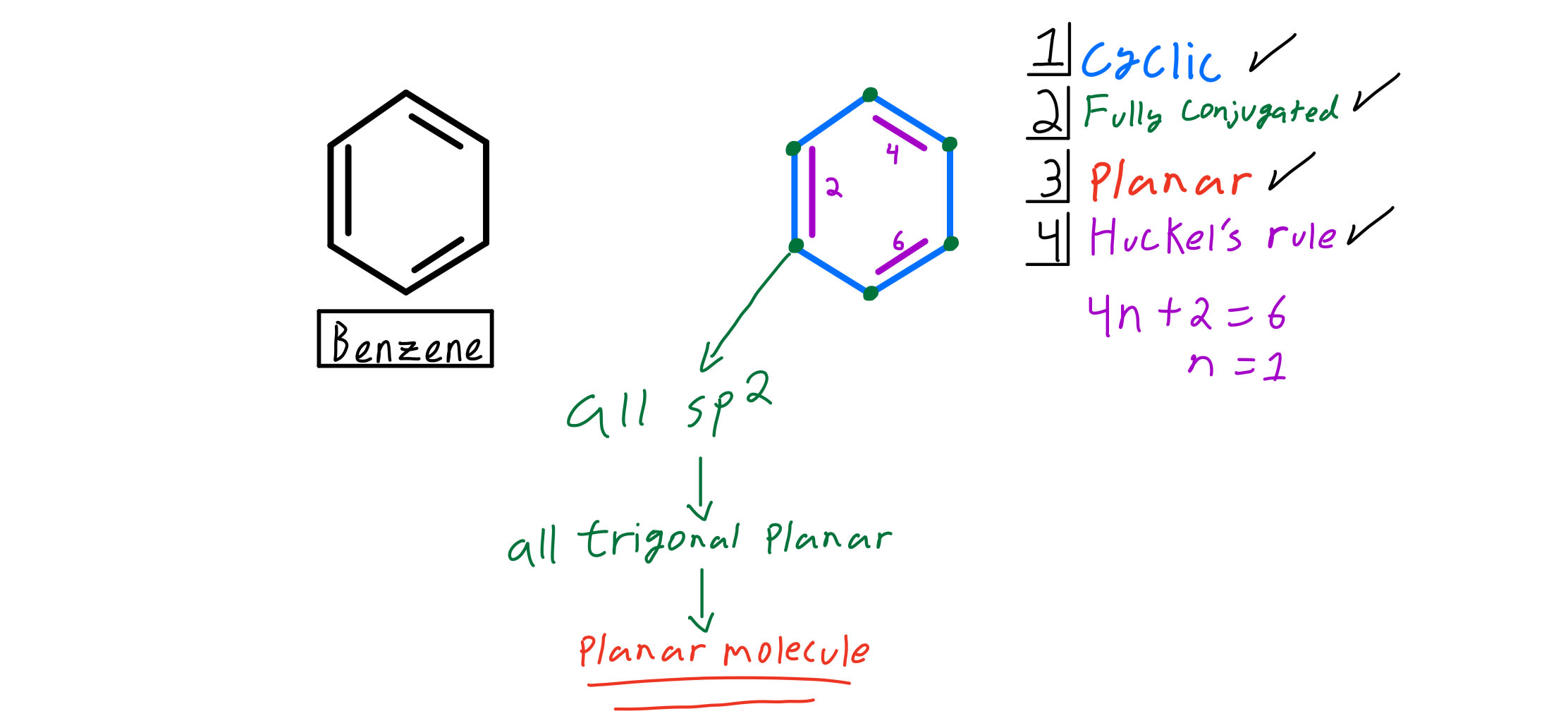



Huckel S Rule Organic Chemistry Video Clutch Prep



Illustrated Glossary Of Organic Chemistry Term
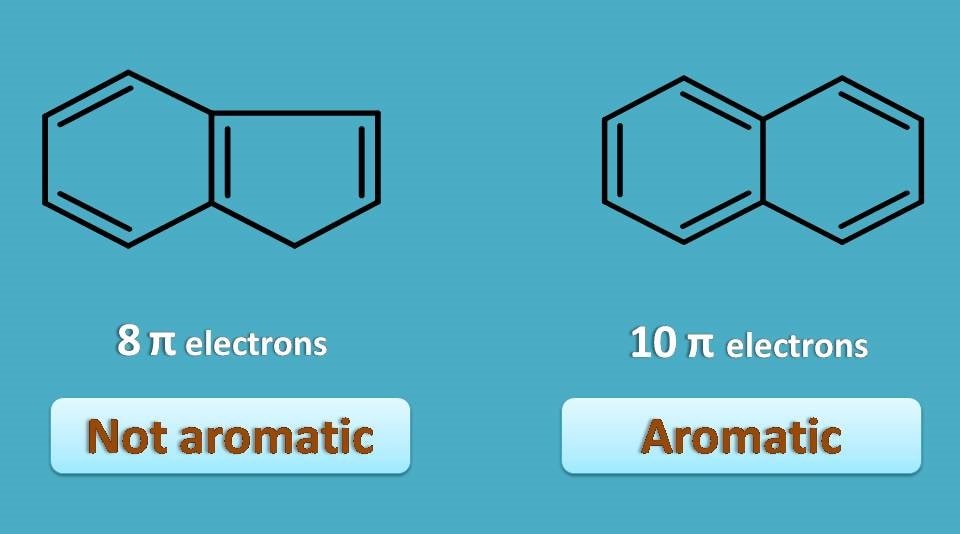



Aromaticity 4 Criteria Every Compound Needs




Mobius Aromaticity Wikipedia
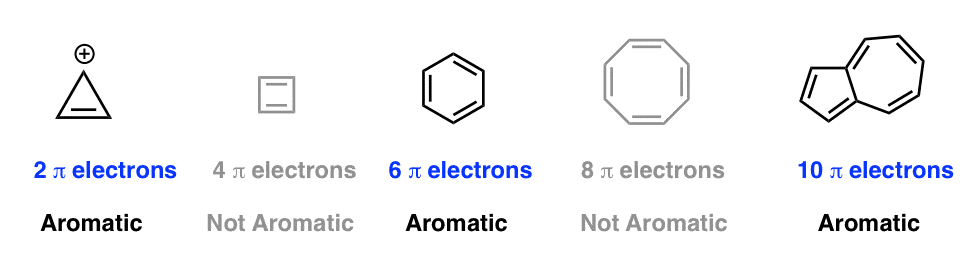



Huckel S Rule What Does 4n 2 Mean Master Organic Chemistry




Huckel S Rule Aromatic And Antiaromatic Compounds Chemistry Steps
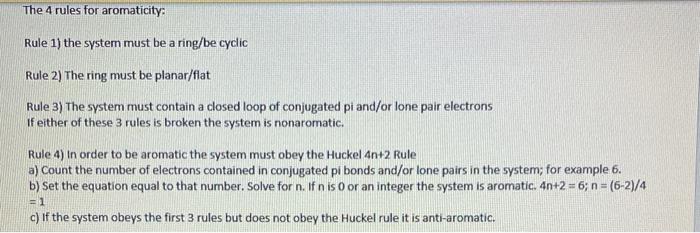



Solved The 4 Rules For Aromaticity Rule 1 The System Must Chegg Com




Electrocyclic Reactions




What Is Huckel S Rule




Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps




The Annulenoscb Ring Openings And The Postulated Anti Aromatic Download Scientific Diagram




Aromatic Hydrocarbons Organic Chemistry Video Clutch Prep



15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts
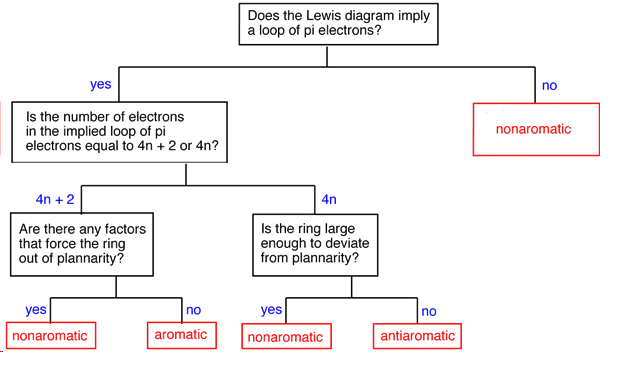



15 3 Aromaticity And The Huckel 4n 2 Rule Chemistry Libretexts




Neet Ug Aromaticity And Huckel Rule Offered By Unacademy
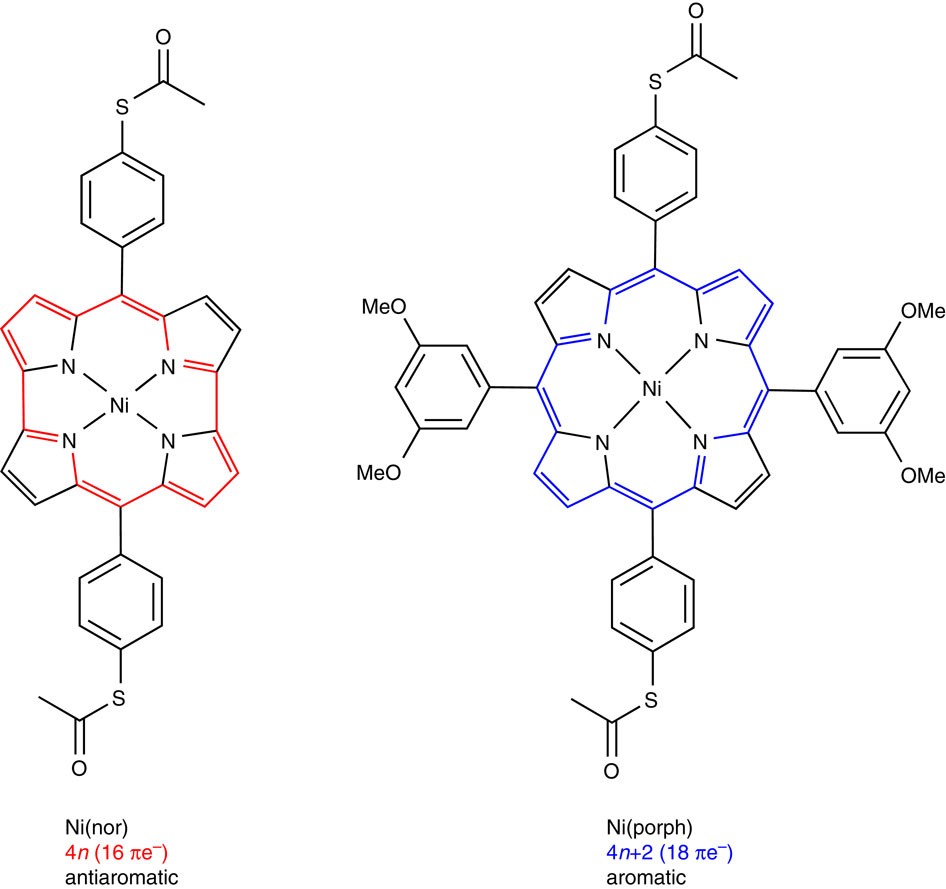



Highly Conducting Molecular Circuits Based On Antiaromaticity Nature Communications



Pdf Aromaticity Antiaromaticity Homoaromaticity And The Huckel 4n 2 Rule



A Disrotatory 4n 2 Electron Anti Aromatic Mobius Transition State For A Thermal Electrocyclic Reaction Henry Rzepa S Blog




What Is An Aromatic Compound Definition Example Video Lesson Transcript Study Com



Illustrated Glossary Of Organic Chemistry Term




Hu Ckel 4n 2 Rule




Identifying Aromatic Compounds Organic Chemistry



Aromatic Stability I Video Khan Academy



Rules For Aromaticity The 4 Key Factors Master Organic Chemistry




13 6 Aromaticity Organic Chemistry Ii
.jpg?revision=1&size=bestfit&width=317&height=254)



Huckel S Rule Chemistry Libretexts




Huckel S Rule Aromatic And Antiaromatic Compounds Chemistry Steps




Aromaticity The Huckel 4 N 2 Rule And Magnetic Current Zhao 17 Chemistryselect Wiley Online Library




Which Of The Following Signifies An Aromatic Compound
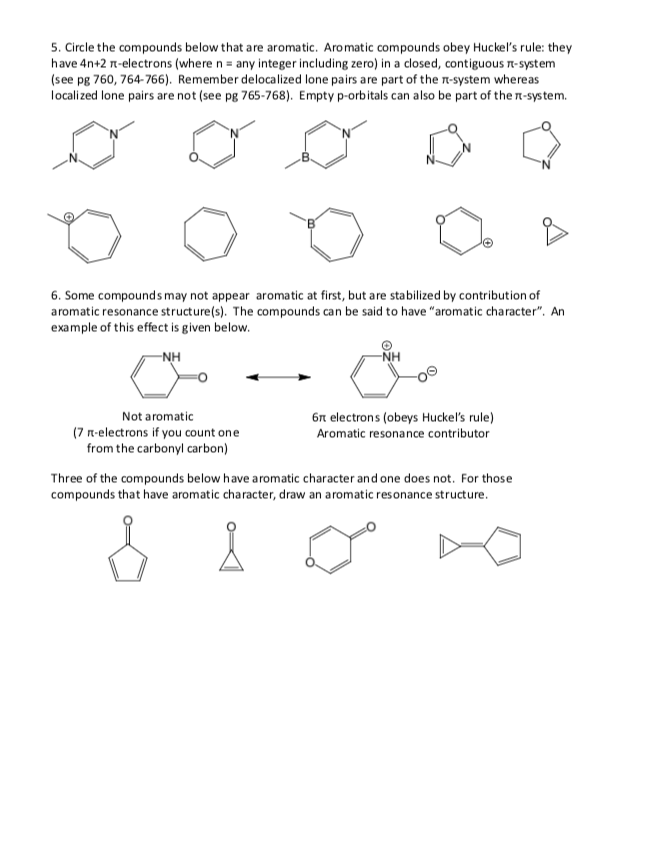



Solved 5 Circle The Compounds Below That Are Aromatic Chegg Com



Illustrated Glossary Of Organic Chemistry Term



1




Aromatic Compounds Ppt Video Online Download




Aromaticity Huckel S Rule Crack Chemistry
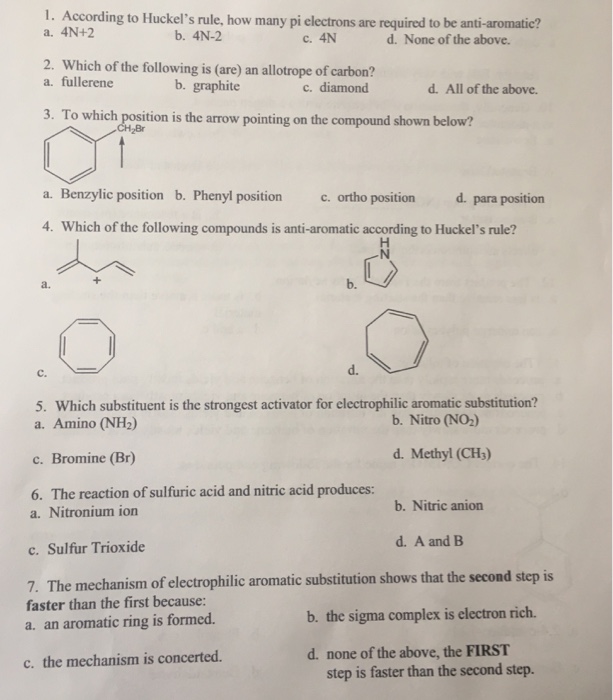



Solved 1 According To Huckel S Rule How Many Pi A 4n 2 Chegg Com
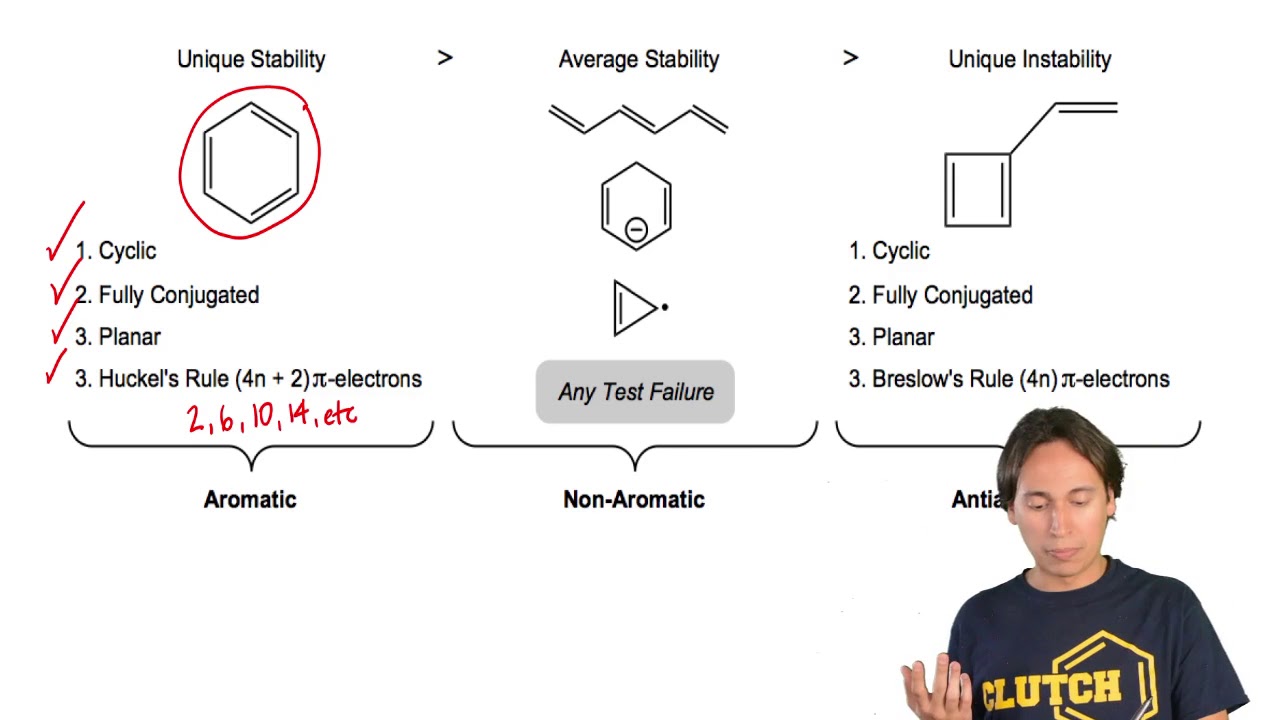



Huckel S Rule Organic Chemistry Video Clutch Prep



1




Woodward Hoffmann Rules Wikipedia
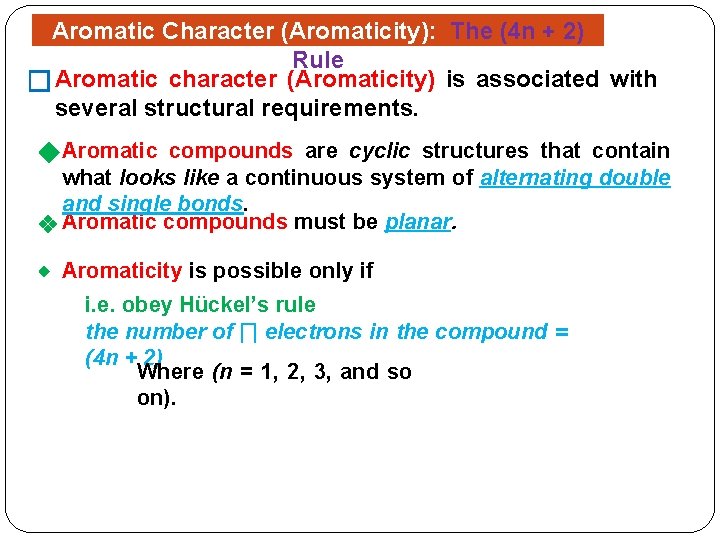



Organic Chemistry Chem 145 Chemistry 2 Credit Hrs




Huckel S Rule Definition Formula And Examples



Illustrated Glossary Of Organic Chemistry Term



Aromatic Antiaromatic Or Nonaromatic Huckel S Rule 4n 2 Heterocycles Aromaticity Lesson Plan Spiral




15 3 Aromaticity And The Huckel 4n 2 Rule Pdf 15 3 Aromaticity And The Huckel 4n 2 Rule Objectives After Completing This Section You Should Be Course Hero




15 3 Aromaticity And The Huckel 4n 2 Rule Pdf 15 3 Aromaticity And The Huckel 4n 2 Rule Objectives After Completing This Section You Should Be Course Hero
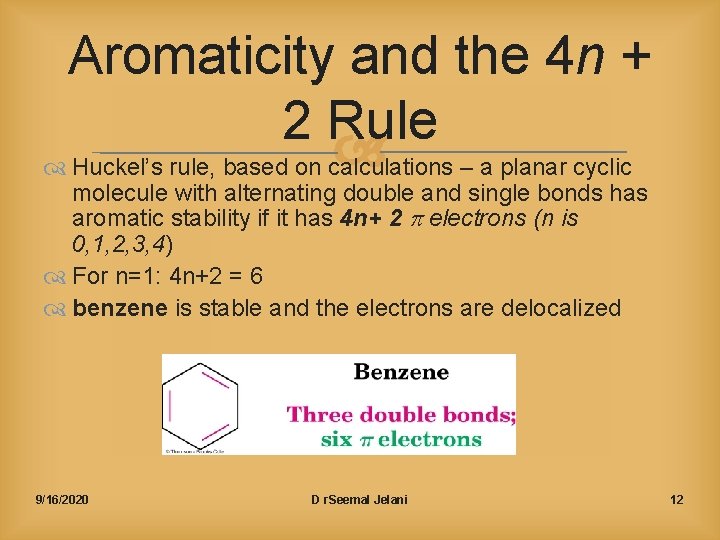



Benzene And Aromatic Compounds Chem160 916 D R
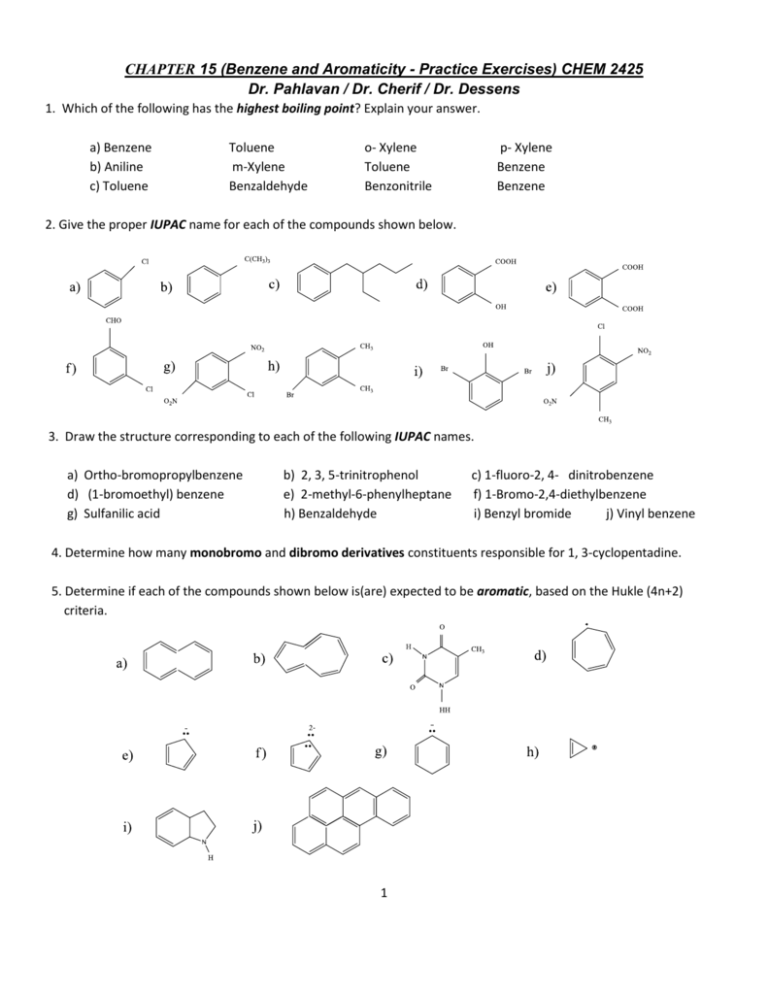



Chapter 15 Benzene And Aromaticity
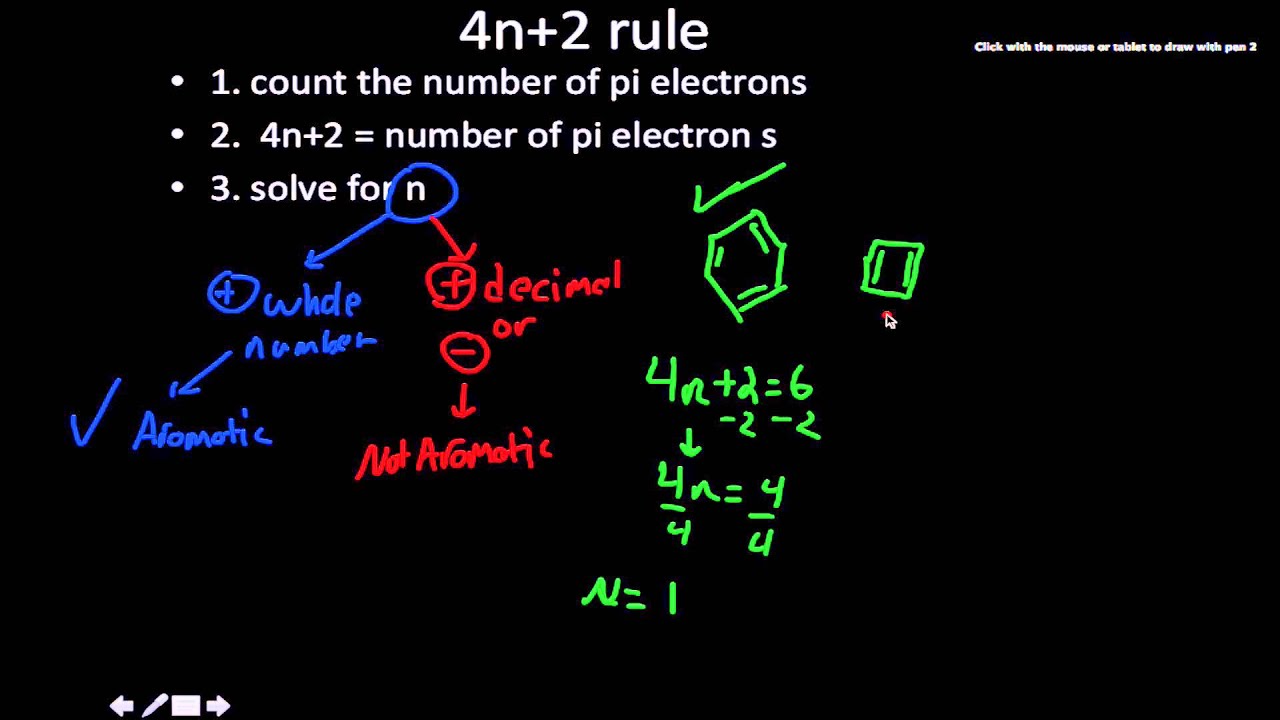



4n 2 Rule Youtube




Huckel S Rule Aromatic And Antiaromatic Compounds Chemistry Steps
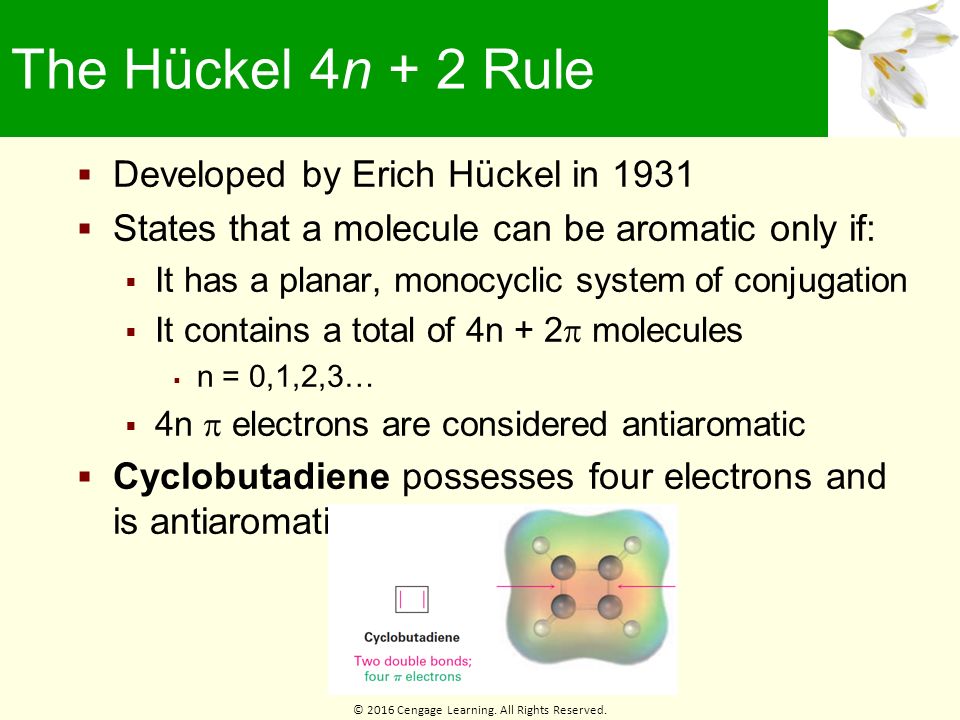



Chapter 15 Benzene And Aromaticity Ppt Download




Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps




Solved 6 Provide An Example Of A 4n And 4n 2 System With Chegg Com




The Criteria For Aromaticity Mcc Organic Chemistry




Benzene And Aromaticity Ppt Video Online Download




Huckel S Rule Definition Formula And Examples




Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps



0 件のコメント:
コメントを投稿